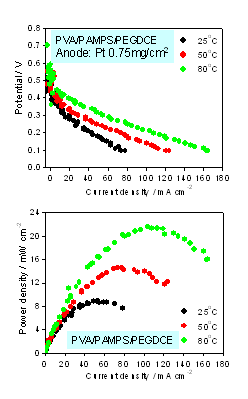


In PEFC using reformed hydrogen gas as fuel, the anode catalysts are susceptible to CO poisoning due to low operation temperatures in the range 80-90 ℃. In order to cope with this problem, CO tolerant anode catalysts are desired and being developed for these 10-20 years. Pt-Ru alloy catalysts are the most promising candidates as practical catalysts, but the cost and low natural abundance are problems. Moreover, even Pt-Ru was not able to sustain 100 ppm CO that would occur during the start-up period of reformers. Alternatives to Pt-Ru were pursued more than 10 years worldwide, but unfortunately with very little success.
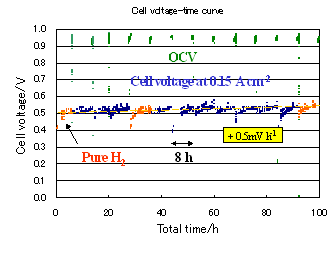
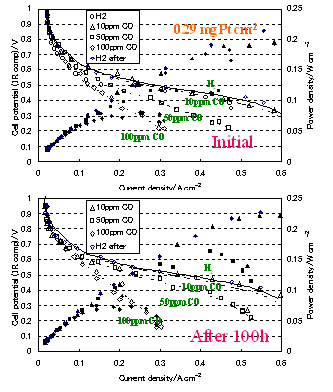
We have developed new CO tolerant anode catalysts that were synthesized by heat-treating the mixture of platinum precursor and organic metal complexes on carbon particles. Single fuel cell experiments with H2 gas containing CO as the anode gas and air as the cathode gas at 70 ℃ showed that, these catalysts showed 100 ppm level CO tolerance in operation conditions (2 patents pending).
Among small fuel cells, direct methanol fuel cell (DMFC) with its power density potentially higher than that of lithium secondary battery is a promising candidate as the power supply for portable applications such as cell phones, PDAs and laptop computers. So far, the planar or chip type air breathing DMFCs are competitively being developed worldwide for portable applications.
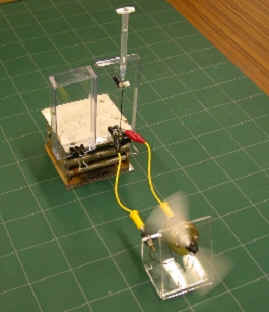
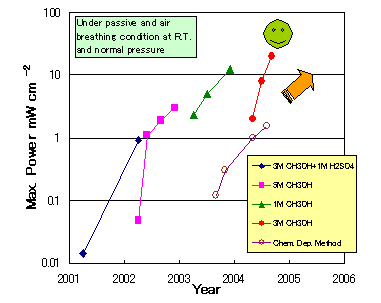
We have been developing a novel prototype of DMFCs utilizing tubular polymer electrolyte membranes and successfully demonstrated them for applications in portable devices. In order to improve the performance of this micro-tubular DMFC, novel catalysts and membranes are under development. Last year, the micro-tubular DMFC single cell where the catalyst layer is formed by the brushing method reached to the power density of 12 mW cm-2 under passive and air breathing conditions at ambient temperature and pressure. This year, the peak power density achieved 20 mW cm-2. This steady progress suggests that the micro-tubular DMFC reaches to the practical level in terms of power density.
Furthermore, as related technology, a novel hydrogen electrode consisting of micro tubular electrolytes was successfully developed. Compared to conventional glass body hydrogen electrodes using platinized Pt electrodes, this micro reference electrode indicates extremely stable potential with less disturbances to electrochemical systems. It is usable as an ideal micro reference electrode not only for fuel cells but also for the micro-analytical and electrochemical systems. (Patent pending)
1. T. Okada: "Polymer Electrolyte Membrane Fuel Cell Systems (PEMFC): Effect of Ionic Contaminants" in "Handbook of Fuel Cells - Fundamentals, Technology and Applications" Vol. III, Chap. 48, pp. 627-646 (Eds. by V. Vielstich, A. Lamm, and H. Gasteiger), John Wiley & Sons, England (2003).
2. N. I. Wakayama, T. Okada, and L. B. Wang: "The Effects of Permanent Magnets on Oxygen Reduction Reaction and Their Application in Polymer Electrolyte Fuel Cells (PEFC)" in "Focus on Electrochemistry Research", Chap. 1, pp. 1-35 (Ed. M. Nunez), Nova Science Publishers, Inc., New York (2005).
3. T. Okada, M. Saito, and K. Hayamizu: "Ion Exchange and Transport Characteristics of Perfluorinated Polymer Electrolyte Membranes for Fuel Cells" in "Electroanalytical Chemistry Research Developments", Chap. 1, pp. 7-84 (Ed. by P. N. Jiang), Nova Science Publishers, Inc., New York (2007).
4. J. Qiao and T. Okada: "Hydrocarbon Polymer Electrolytes for Fuel Cell Applications", in "Electroanalytical Chemistry Research Developments", Chap. 2, pp. 85-134 (Ed. by P. N. Jiang), Nova Science Publishers, Inc., New York (2007).
5. T. Okada: "Impurity Effects on Electrode Reactions in Fuel Cells" in "Proton Exchange Membrane Fuel Cell Durability" Part II, Chap. 2.4 (Eds. by M. Inaba, T. J. Schmidt, and F. N. Buchi), Springer, New York (2009).
6. T. Okada and M. Kaneko eds. "Molecular Catalysts for Energy Conversion", Springer Series in Material Science 111, Springer-Verlag, Heidelberg (2009).